
Quality Standards for Hormone Manufacturers in India — What Makes Sanzyme Stand Out
Introduction The global demand for hormone therapies continues to grow, and India has emerged as a major hub for hormone API and finished-dose production. For















Currently we operate in emerging markets with our distribution partners and in house brands.
We are open for Out Licensing / Co-development / other models of collaboration, as we expand our footprints to highly regulated markets.
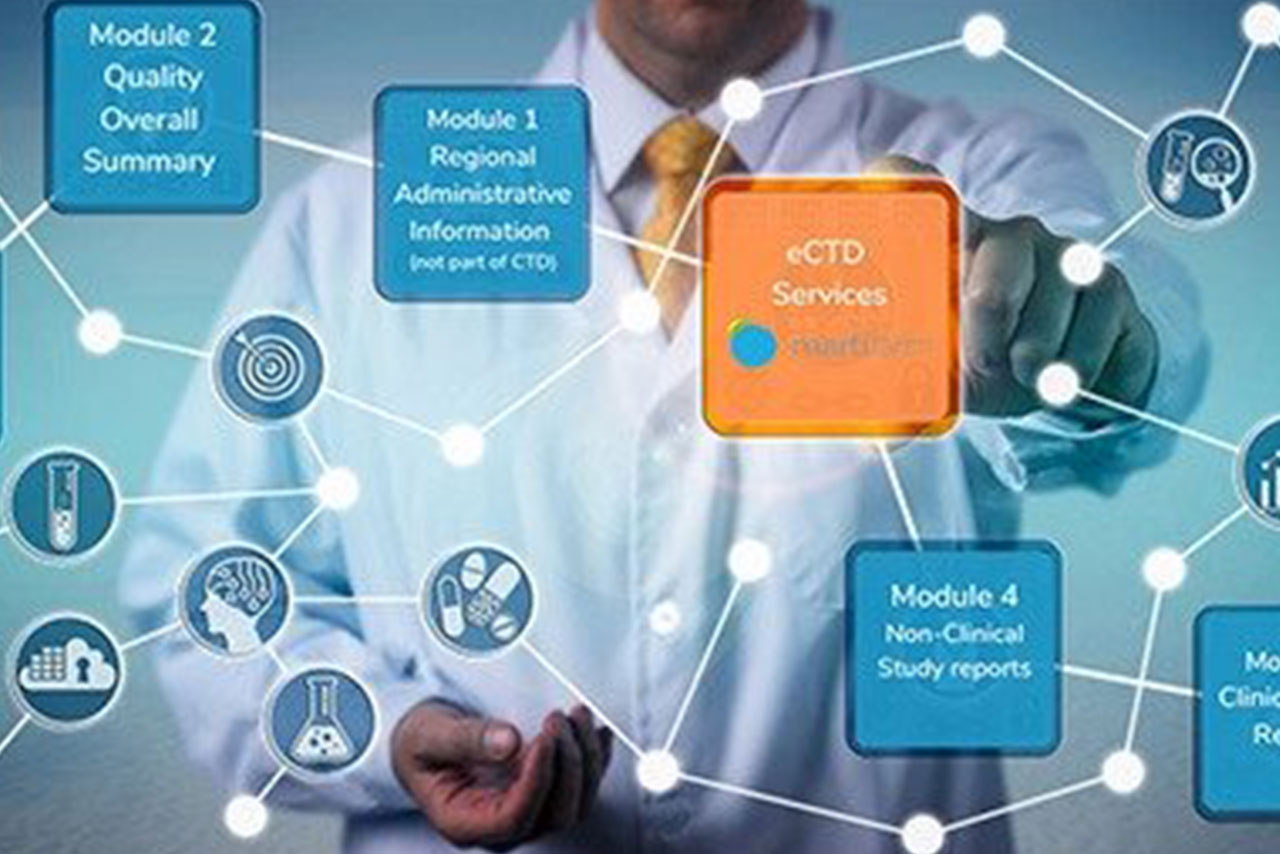
We at Sanzyme are fully aware and prepared of dynamic regulatory landscape in the segment of Biologics/Biosimilars.
Armed with EU GMP & PIC/s for our Finished Formulations, we offer our e-CTD to cater to global regulatory requirements.
Navneet Battu (Vice President – International Business) (navneet.battu@sanzyme.com)
Naimesh Prajapati (naimesh.prajapati@sanzyme.com) and Navneet Battu (Vice President – International Business) (navneet.battu@sanzyme.com)
Rahul Jagadishan (rahul.j@sanzyme.com) and Navneet Battu (Vice President – International Business) (navneet.battu@sanzyme.com)
Rahul Jagadishan (rahul.j@sanzyme.com) and Navneet Battu (Vice President – International Business) (navneet.battu@sanzyme.com)
Pundeep Chaparala (pundeep.chaparala@sanzyme.com) and Navneet Battu (Vice President – International Business) (navneet.battu@sanzyme.com)
Navneet Battu (Vice President – International Business) (navneet.battu@sanzyme.com)


Introduction The global demand for hormone therapies continues to grow, and India has emerged as a major hub for hormone API and finished-dose production. For

Introduction Assisted reproduction (or assisted reproductive technology, ART) has revolutionized the way couples with fertility challenges can pursue parenthood. At the heart of most ART

Infertility is a growing concern worldwide, and assisted reproductive technologies (ART) such as in vitro fertilization (IVF) have given hope to millions of couples. Among
October 30, 2025• bySanzyme_Admin
October 30, 2025• bySanzyme_Admin

Since 1969, when we entered into a Joint Venture with Sankyo (now Daiichi-Sankyo) to make gonadotropins to export to Japan, we’ve been at the forefront of biological hormones, and today we have the necessary technological foundation to do path breaking work.